Image of the month
Spooky urolith centers
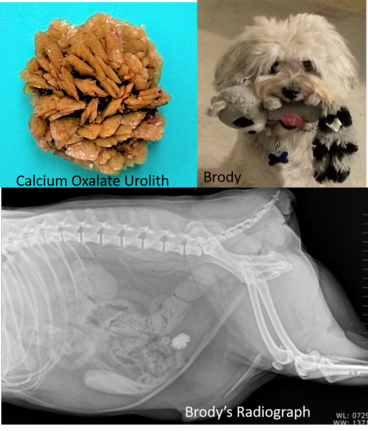
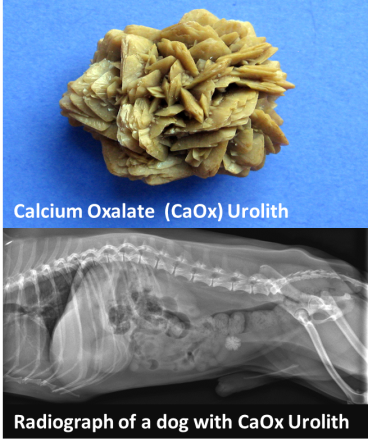
This month we are focusing on the interesting, strange and scary things we have found in the center of uroliths.
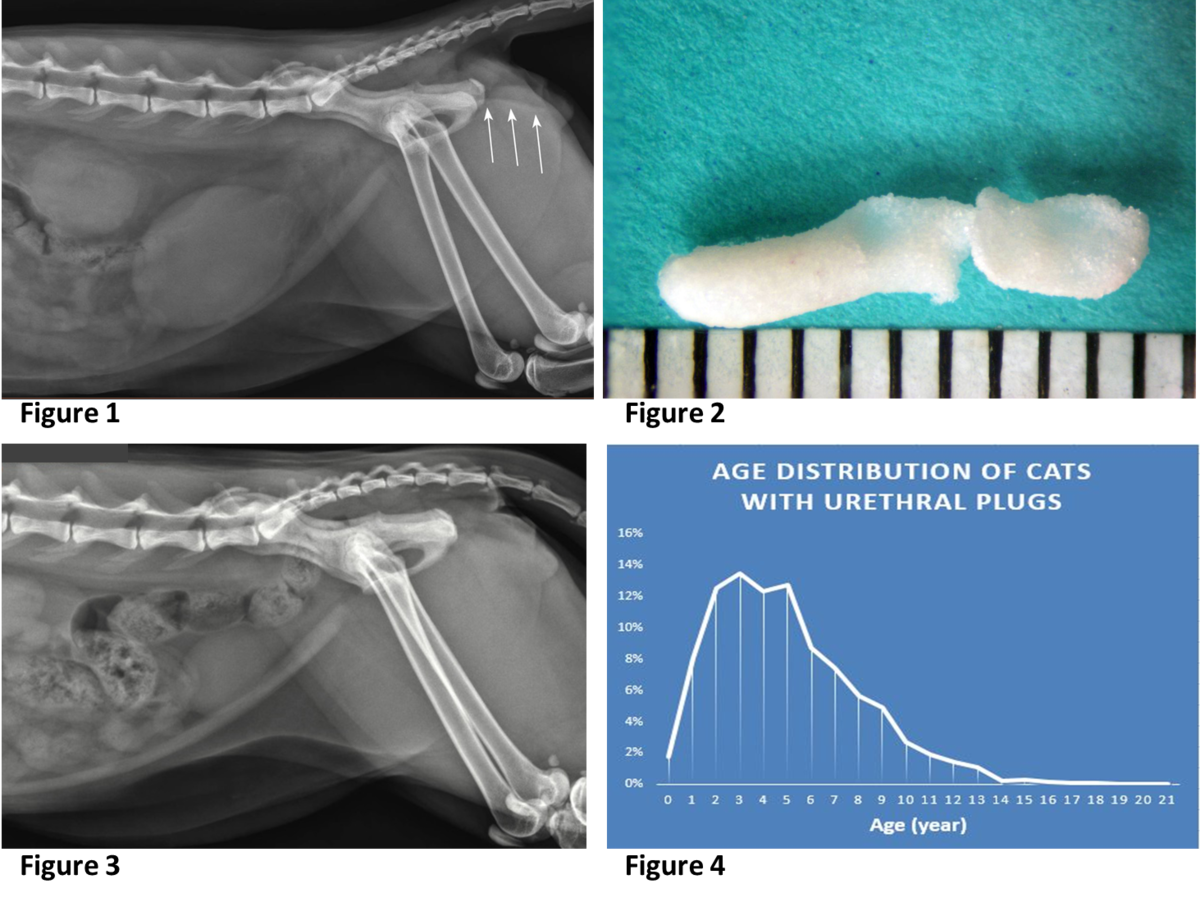
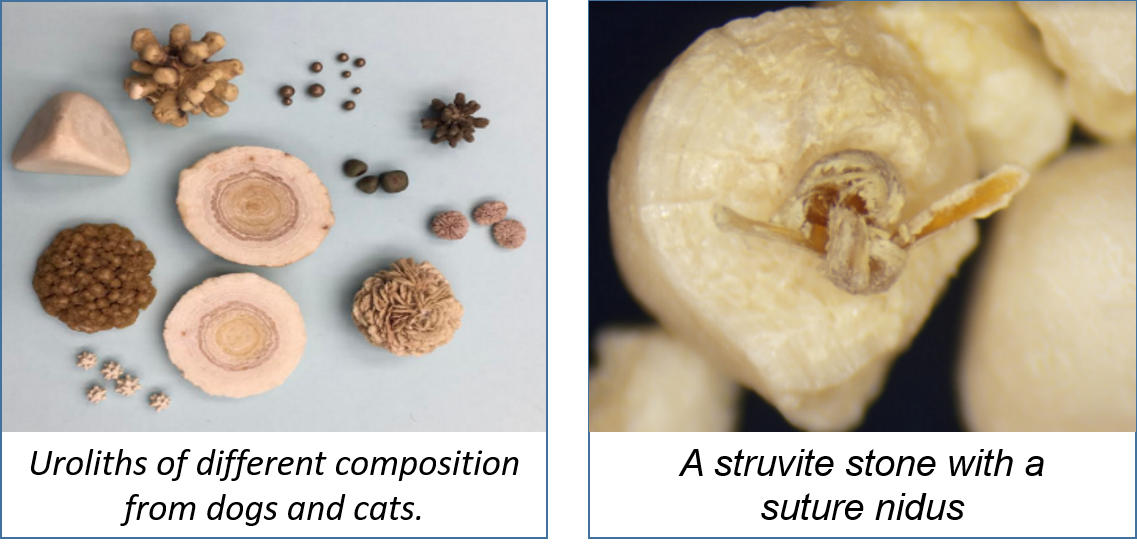
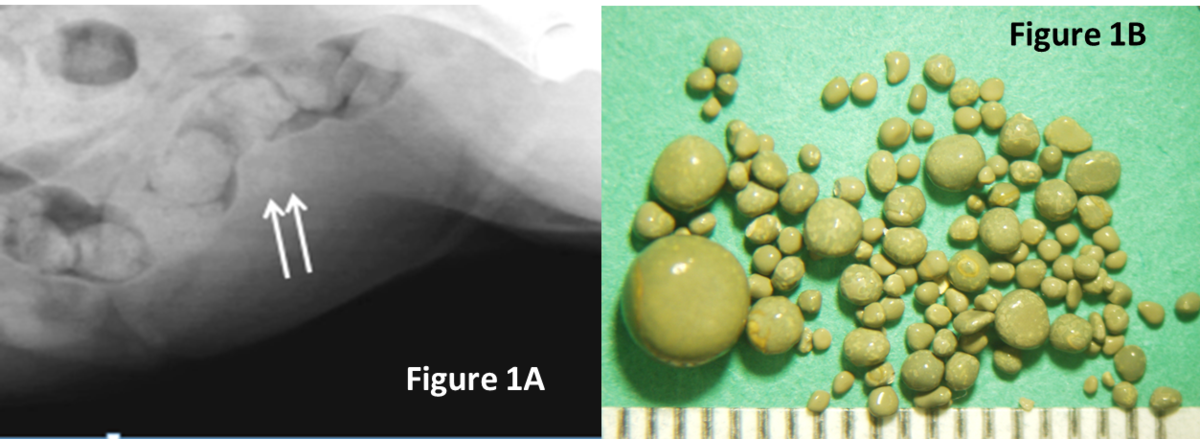
- Suture was identified in approximately 10% of recurrent stones. When closing the bladder after a cystotomy, minimize placing suture in the bladder lumen to reduce its contribution to stone recurrence. Image A
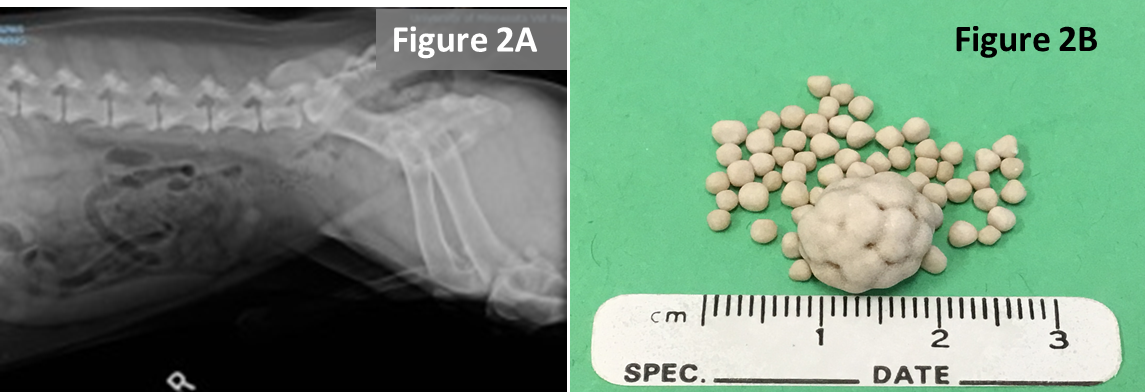
- Plant awns/material are common in the western United States and sometimes enter the bladder and can become encrusted with mineral. Image B
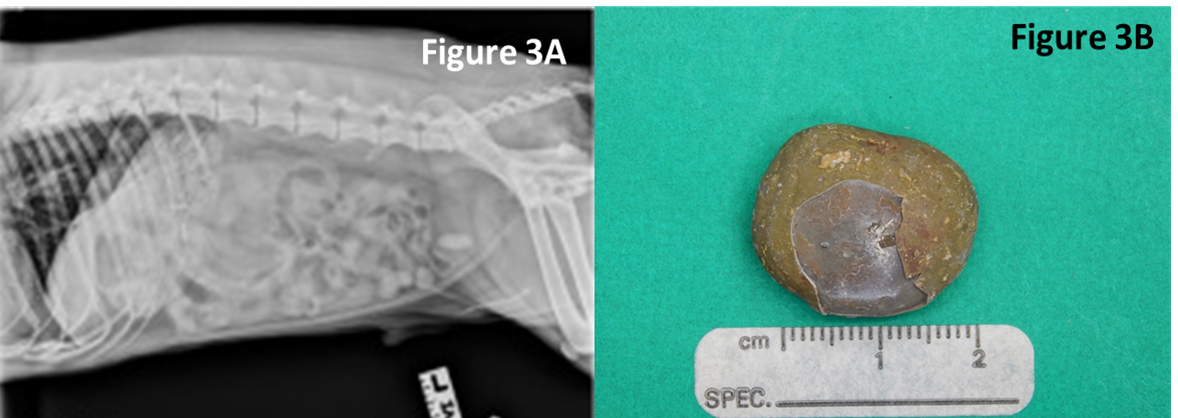
- Toothpick - a migrating toothpick could have been swallowed by the pet because it held a tasty Halloween treat. In this case, the toothpick pierced the intestine, traveled to the bladder and initiated stone formation. Image C
- Fleas, louse, and other insects may climb up the urethra and become entombed in stones. Image D

What do all of these spooky invaders have in common?
Heterogeneous nucleation: the process where crystallization is enhanced by initiating over foreign-preformed surfaces.
What’s in the center of your uroliths?
For complete and accurate urolith analysis – please submit the entire urolith.