Researchers discover promising new target for sarcoma therapies
August 3, 2023
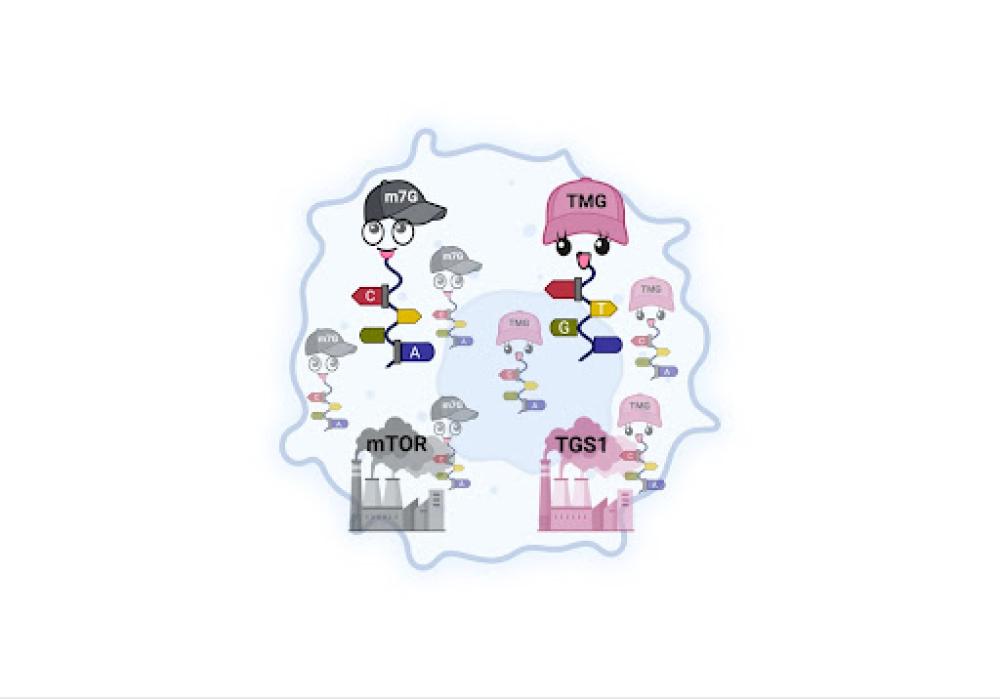
Genetic code is stored in DNA and copied into messenger RNA (mRNA), where it’s made into proteins. All mRNAs work with methyl-7-Guanosine that’s attached to the m5G-cap (represented here by the gray baseball cap), which is created by an enzyme called mTOR. But cancer cells promote another pathway, represented here by the pink cap, in which a different enzyme, called TGS1, converts m7G-caps into different caps, called TMG-caps. These pathways feed sarcoma cells.
Researchers at the University of Minnesota (UMN) College of Veterinary Medicine (CVM) have identified an epigenetic mark that appears to drive tumor growth.
Epigenetic marks are created by enzymes and have the power to silence certain genes and alter the production of certain proteins that influence the growth and development of a living creature. The research team found that neutralizing the enzyme that creates this specific epigenetic mark can make a dog more susceptible to treatment for osteosarcoma and hemangiosarcoma, two aggressive types of cancer.
Sarcomas—a broad family of cancers that affect soft tissues and bones—are rare in humans, making up about 15 percent of childhood cancers and just 1 percent of cancers in adults. The higher incidence of sarcoma in dogs provides a unique opportunity to understand how these cancers behave, and eventually develop and test therapies that could help both species.
Kathleen Boris-Lawrie, a professor in the CVM Department of Veterinary and Biomedical Sciences, who led the study, and her co-author, recent graduate student Dr. Dora Zucko, used CVM’s vast bank of osteo- and hemangio-sarcoma cell lines to identify the new potential target for sarcoma therapies.
They turned their focus to epigenetic marks that sit at the very beginning of messenger RNA (mRNA), called RNA caps. Scientists recognize caps are key players in cell biology, but until now, no one had discovered how they were implicated in sarcoma growth.
The newly discovered epigenetic mark is created by an enzyme called Tri methyl guanosine synthase 1 (TGS1), which makes the enzyme—the mark’s source—a potential target.
In the new study, Boris-Lawrie first discovered that mutated cells do, indeed, use TGS1 to replicate. The team also figured out a potential way to halt TGS1’s cancer-stoking activity.
They found that TGS1 converts select mRNA caps from epigenetic marks called 7-methyl-guanosine to ones called tri-methyl-guanosine (TMG-caps). Rather than following a normal pathway for healthy cellular growth, these TMG-caps gain entry to a rogue, sarcoma-feeding pathway, allowing tumors to grow.
But its trajectory can be reversed.
Boris-Lawrie and Zucko found that they could redirect the source of the TMG-caps—TGS1— away from the rogue pathway that was feeding the tumor, and towards the cell’s common pathway. Then, they used an existing cancer therapy called mTOR inhibitor to block new TGS1 proteins from being made, cutting tumors off from their source.
The study was experimental, and further research will need to determine whether or not using TGS1 as a target could, indeed, make dogs more susceptible to treatment with existing sarcoma therapies.
Another complex question remains: Since the rogue pathway is turned on by the same epigenetic mark that modulates the pathway, what would be the best approach to targeting it?
“It's a kind of chicken or the egg situation,” said Boris-Lawrie.
Read the full study in Nature Cancer Gene Therapy.


