University researchers discover, define RNA pathway that allows HIV-1 to thrive under cell stress
January 27, 2022
ST. PAUL, Minn. — University of Minnesota scientists have defined the method that HIV-1 uses to deceive host cells into allowing the virus to proliferate, according to new research published this month. The discovery identifies a way forward for antiviral drug research to recognize and combat the deception and prevent the spread of the virus that causes AIDS.
The specific mechanism for how HIV-1 persisted during a host cell’s stress-response period had been unclear until now.
Researchers had known that HIV-1 messenger RNAs (mRNA) hijack a host’s machinery to synthesize proteins that cause the cell to make more viral mRNA and to make the viral proteins that amplify the host’s infection. Those newly created viral proteins trigger special host RNAs that sound alarm bells meant to combat stress and prevent the viral RNAs from using its machinery. Still, the researchers knew, the virus continued to find a way.
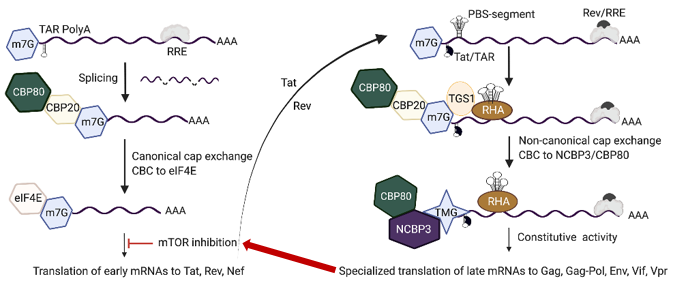
 Now, UMN scientists have found that certain HIV-1 mRNAs can impersonate the alarm bell RNAs while others simply access the same specialized pathway the alarm bells use to synthesize proteins—ensuring the growth of new viral particles. In order to access the specialized alarm-bell pathway, the host cell must confer a license to the viral mRNAs. Now, for the first time, the scientists have defined the license that the HIV-1 mRNAs have accessed—as if obtaining counterfeit travel documents—and determined how they did it.
Now, UMN scientists have found that certain HIV-1 mRNAs can impersonate the alarm bell RNAs while others simply access the same specialized pathway the alarm bells use to synthesize proteins—ensuring the growth of new viral particles. In order to access the specialized alarm-bell pathway, the host cell must confer a license to the viral mRNAs. Now, for the first time, the scientists have defined the license that the HIV-1 mRNAs have accessed—as if obtaining counterfeit travel documents—and determined how they did it.
The findings are significant beyond HIV-1 and have interspecies implications. For one, the research team believes these findings and those from previous studies open the possibility that a similar deception takes place in cancer cells. And the researchers are already looking for the same counterfeit documents in animal retroviruses such as bovine leukemia and equine infectious anemia. Importantly, they are looking at SARS-CoV-2, the virus that causes COVID-19, as well.
Now that the HIV-1 pathway is understood, a team of scientists that includes medicinal chemists can help develop chemicals to block the viral mRNAs from obtaining the counterfeit documents—which would stop the virus from proliferating.
“There still might be HIV-1 infection but there might not be progression to AIDS,” said Professor Kathleen Boris-Lawrie, PhD, the project’s principal investigator. “Because blocking HIV-1 specialized translation takes away the virus’s ability to grow in stressed T cells.”
The counterfeit document the HIV-1 mRNA uses is determined by the ‘cap’ conferred on it by the host cell. Early HIV-1 and regular, nonviral mRNA receive a cap called 7-methyl-guanosine (m7G-cap) during homeostasis—when the cell feels healthy. Late HIV-1 mRNA, spurred by newly synthesized early-HIV-1 proteins, is more complex in shape and so m7G undergoes hypermethylation into tri-methyl-guanosine (TMG) cap, after which it is conferred a unique combination of cap-binding proteins. That same cap-binding complex is used by mRNA the host creates to facilitate recovery when the cell is under duress. Under cell culture analysis, the researchers found both mRNAs—the ones for recovery and the late HIV-1—make it through the translation process and continue protein synthesis.
If scientists can determine how to block the late HIV-1 mRNA from wearing TMG caps, they expect that would cause those viruses to stop proliferating—which would be a significant victory for people living with HIV-1.
“Since HIV-1 requires the specialized translation pathway to thrive during the host’s stress-response period, blocking its TMG license impedes specialized translation,” Boris-Lawrie said. “That diminishes the virus's proliferation and attenuates the infection.”
The study, authored by Boris-Lawrie, PhD, in the Department of Veterinary and Biomedical Sciences, was published this month in the journal Proceedings of the National Academy of Sciences. Key to the study were collaborators from the University of Missouri, including Associate Professor of Biochemistry Xiao Heng. The study was funded by a grant from the National Institutes of Health.
Future research, the scientists say, should focus on how to construct ‘blockers’ that recognize the shape of the late HIV-1 mRNA and prevent it from gaining the ability to thrive during the host cell’s stress-response period.